Yingxia Zong,YuanyuanZhou,*MinggangJu,Hector F.Garces, Amanda R. Krause,Fuxiang Ji, Guanglei Cui, Xiao Cheng Zeng, Nitin P. Padture,* and Shuping Pang*
Abstract: Methylamine-induced thin-film transformation at room-temperature is discovered, where a porous, rough, polycrystalline NH 4 PbI 3 non-perovskite thin film converts stepwise into a dense, ultrasmooth, textured CH 3 NH 3 PbI 3 perovskite thin film. Owing to the beneficial phase/structural development of the thin film, its photovoltaic properties undergo dramatic enhancement during this NH 4 PbI 3 -to- CH 3 NH 3 PbI 3 transformation process. The chemical origins of this transformation are studied at various length scales.
H ybrid organic–inorganic perovskites (HOIPs) are a class of materials with the general formula ABX 3 , where A is an organic cation, X is a halogen anion, and B is typically Pb 2+ . [1] Their unique hybrid crystal structures have endowed these amazing HOIP materials interesting and unconventional properties, with great promise in a wide range of applica-tions. [1,2] In particular, there has been a surge of interest in using HOIPs in solar cells. [2,3] Thus, enormous amount of effort is being devoted towards studying these materials, invoking new concepts in inorganic–organic hybrid chemis-try. [4] Here, we report the discovery of a unique room-temperature thin-film transformation of NH 4 PbI 3 -to-CH 3 NH 3 PbI 3 induced by methylamine (CH 3 NH 2 ) gas, and provide insights into its chemical origins.
The structure of ABX 3 is determined empirically by the Goldschmidt tolerance factor, [1] t=(r A +r X )/{ffififfi 2p (r B +r X )}, where r A , r B , and r X are the radii of the A, B, and X, respectively. For NH 4 PbI 3 , t is 0.76. [5] In this context, at ambient temperature, instead of a stable 3D perovskitestructure, NH 4 PbI 3 usually exhibits a 1D non-perovskite structure (orthorhombic, space group Pnma). [6] This reduced structural dimensionality results in the relaxation of the band gap and, thus, NH 4 PbI 3 appears yellow with a large indirect band gap >2 eV, as revealed by the Tauc plot (Figure S1 in the Supporting Information (SI)) and the band structure (Figure S2 in the SI) calculated using density functional theory (DFT). Furthermore, the 1D crystal structure of NH 4 PbI 3 energetically favors the formation of high aspect ratio structures, instead of dense thin films, from simple “one-step” solution deposition. Such (opto)electronic and morpho-logical characteristics suggest that the solution-grown NH 4 PbI 3 is not a very useful material for single-junction solar cells. [7] Here we have discovered that a rough, porous, and polycrystalline NH 4 PbI 3 non-perovskite thin film trans-forms into an ultrasmooth, dense, and textured CH 3 NH 3 PbI 3 (MAPbI 3 ) HOIP thin film by using a methylamine-induced conversion–healing process. As a result, the photovoltaic (PV) properties of the thin film undergo dramatic enhance-ment. The transformation of NH 4 PbI 3 was first observed in situin bulk crystals using an optical microscope, as shown in Figure 1. The starting solution-grown NH 4 PbI 3 crystal exhib-its a rod-like morphology with a light-yellow color (Fig-ure 1A). Once methylamine (CH 3 NH 2 ) gas is introduced, the crystal immediately turns black, with its rod-like morphology.

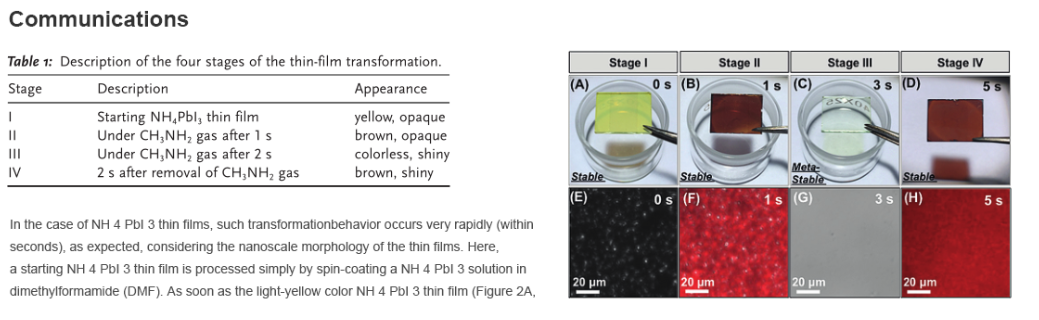
Figure 2. A–D) Photographs and E–H) photoluminescence maps (superimposed on optical microscope images) of the thin film at the four different stages described in Table 1.
Stage I) is exposed to CH 3 NH 2 gas, it darkens within one second (Figure 2B, Stage II), and it becomes bleached after another second (Figure 2C, Stage III). The thin film is then removed from the CH 3 NH 2 gas to the ambient, and a brown shiny thin film readily appears (Figure 2D Stage IV). Figures 2E–H show in situ photoluminescence (PL) maps superimposed on the optical images of the thin films at Stages I–IV. The starting NH 4 PbI 3 thin film appears non-luminescent with microscopic defects (Figure 2E, Stage I). After 1 sexposure to CH 3 NH 2 gas, spots of PL appear (Figure 2F, Stage II). After 2 s, the PL signal vanishes completely (Figure 2G, Stage III), and the defects, as seen in Stages I and II, disappear as well. Finally, when the CH 3 NH 2 gas is removed, PL signal is recovered rapidly (Figure 2H, Stage IV) within 2 s. Compared with Stage II, the PL map at Stage
IV is obviously more uniform. The thin films at all stages were further studied using ex situ UV/vis spectroscopy and X-ray diffraction (XRD). Note that since the colorless film at Stage III is only stable under CH 3 NH 2 gas, its UV/vis spectrum and XRD pattern (Figure S3 in the SI) were acquired using special enclosures containing CH 3 NH 2 gas.
Copyright © Zhengzhou CY Scientific Instrument Co., Ltd. All Rights Reserved Update cookies preferences
| Sitemap | Technical Support: 